Cell-Free DNA
Cancer early diagnosis based on cell-free methylated DNA immunoprecipitation-sequencing and whole genome sequencing
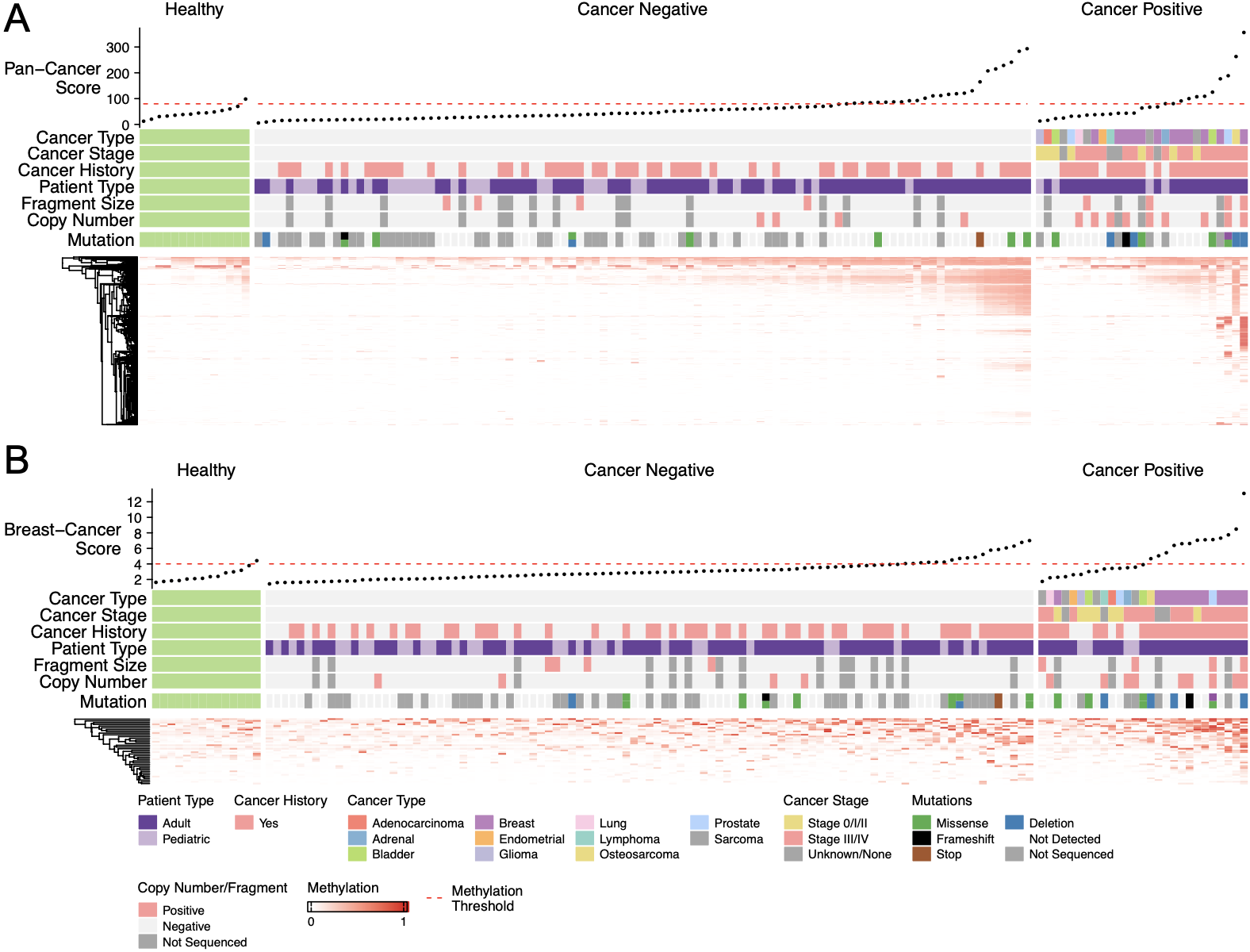
People with Li-Fraumeni syndrome harbor a germline pathogenic variant in the tumor suppressor gene TP53 and face a near 100% lifetime risk of developing a wide spectrum of, often multiple, cancers. TP53 mutation carriers routinely undergo intensive surveillance protocols which, although associated with significantly improved survival, are burdensome to both the patient and the health care system. Liquid biopsy, the analysis of cell- free DNA fragments in bodily fluids, has become an attractive tool for a range of clinical applications, including early cancer detection.
In this project, we assess the efficacy of a multi-modal liquid biopsy assay that integrates a targeted gene panel, shallow whole genome, and cell-free methylated DNA immunoprecipitation sequencing for the early detection of cancer in a cohort of Li- Fraumeni syndrome patients. Our integrated analysis was able to detect a cancer-associated signal in 79.4% of samples from patients with active cancer, a 37.5% – 58.8% improvement over each individual analysis. Through analysis of patient plasma at cancer negative timepoints, we were able to detect cancer-associated signals up to 16 months prior to occurrence of cancer as detected by conventional clinical modalities in 17.6% of TP53 mutation carriers.